The analysis revealed a heightened risk of neurological disorders in patients who had been administered two RSV vaccines.
View pictures in App save up to 80% data.
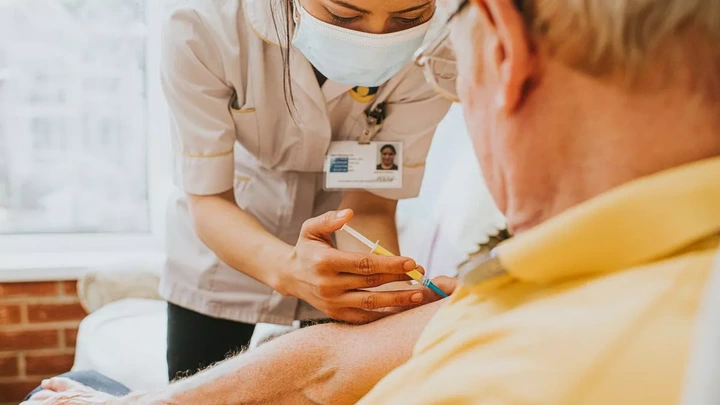
In a crucial health update, new research has prompted the FDA to require GSK and Pfizer's RSV vaccines carry warnings of Guillain-Barré syndrome (GBS) risks. On Tuesday, January 7, it was reported that after a review of post market data, concerns were raised about increased GBS risks in patients who had received either Arexvy or Abrysvo vaccines from the respective pharmaceutical giants.
While only Pfizer’s Abrysvo is available through the NHS, and UK packaging already includes the warning, the update reinforces the importance of staying informed about potential serious side effects. The UK leaflet categorises GBS as a "rare" yet serious condition possibly effecting up to one in 1,000 people who have had the vaccine, especially those over 60.
Regarding GBS, this neurological condition can be quite serious, affecting the nerves and often requiring hospitalization for an extended period, sometimes lasting weeks or months. It can impact various nerve functions, including those related to sensation, movement, and even heart rate, with the first signs usually appearing in the limbs.
View pictures in App save up to 80% data.
Keep an eye out for symptoms such as strange sensations like tingling or numbness in the hands and feet, which may progress to muscle weakness and problems with joint movement. Patients may also suffer from severe nerve pain in the legs or back, as well as challenges related to breathing, facial muscle control, and vision issues, including difficulties swallowing or experiencing double vision.
The symptoms of this condition can significantly deteriorate within the initial month, possibly resulting in paralysis affecting the legs, arms, or facial muscles. Treatment typically requires extended hospital admissions for immunotherapy administered through IV or plasma exchange, in addition to pain relief measures. In severe cases where respiratory problems escalate, some patients may require the use of a ventilator.
The NHS has observed that most individuals are able to walk within six months and typically make a full recovery within a year post-hospitalisation. Although the precise origins of GBS remain elusive, it's thought to be an autoimmune response where the immune system mistakenly attacks the peripheral nervous system.
A study conducted by the FDA, which monitored Medicare beneficiaries aged 65 and older from May 2023 to June 2024, revealed an increased risk of Guillain-Barré Syndrome (GBS) within 42 days following vaccination. Nevertheless, the findings were considered inadequate to establish a definitive "causal relationship." In the end, the agency stated: "The advantages of receiving Abrysvo and Arexvy vaccines still surpass the associated risks."

View pictures in App save up to 80% data.
A representative from Pfizer stated: "In line with our commitment to medicine and vaccine safety, Pfizer implements comprehensive procedures to fulfill its regulatory obligations by diligently monitoring, reporting, and analyzing all adverse events. We gather pertinent information to evaluate any emerging safety risks linked to the RSV (bivalent, recombinant) vaccine. Our pharmacovigilance initiatives, along with adherence to quality and safety regulations, involve close collaboration with the MHRA in the UK, which independently oversees the safety profile of our vaccine."